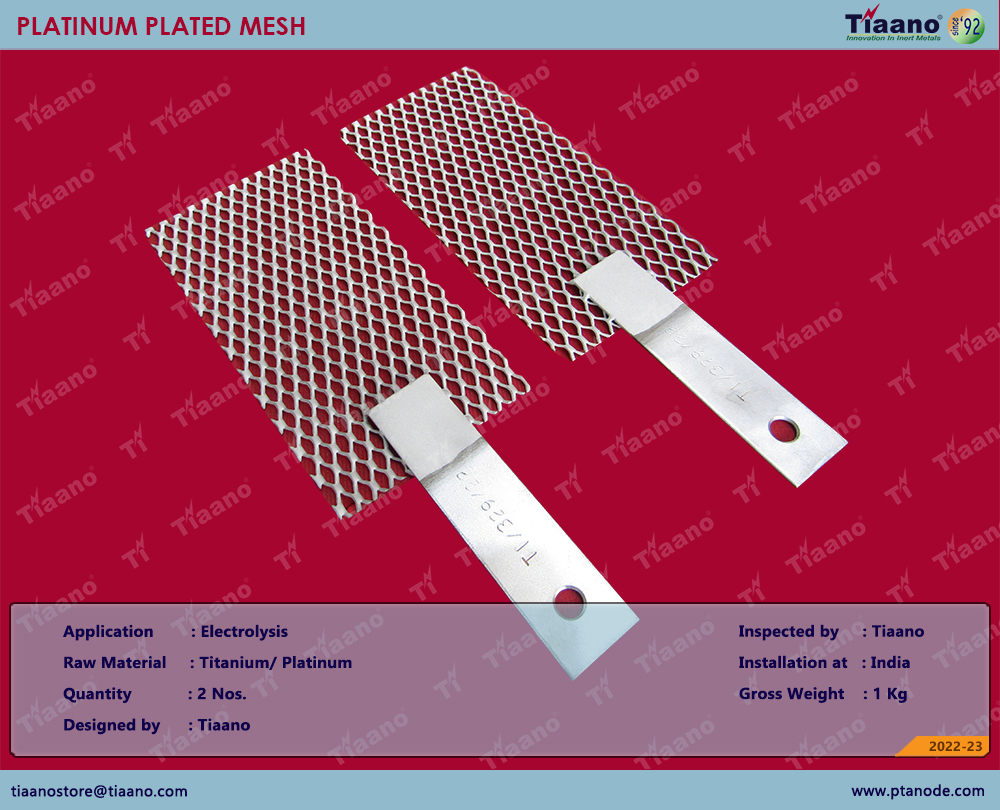
Electrolysis is defined as a process of decomposing ionic compounds into their elements by passing a direct electric current through the compound in a fluid form. The cations are reduced at cathode and anions are oxidized at the anode. The main components that are required for conducting electrolysis are an electrolyte, electrodes, and some form of external power source is also needed. Additionally, a partition such as an ion-exchange membrane or a salt bridge is also used but this is optional. These are used mainly to keep the products from diffusing near the opposite electrode.
For example, when electric current, is, passed through molten sodium chloride, the sodium ion is attracted by the cathode, from which, it takes an electrode and becomes a sodium atom.
Chloride ion reaches the anode, gives its electron, and become chlorine atom to form chlorine molecule.
Na+(in electrolyte) + e–(from cathode) → Na …. At Cathode
Cl–(from electrolyte) → e– + Cl → Cl2 …. At Anode
Electrolysis process, while useful to get elemental forms from compounds directly, it can also be used indirectly in the metallurgy of alkali and alkaline earth metals, purification of metals, deposition of metals, preparation of compounds etc
Electrolysis Applications:--
1) Determination of equivalent eight of substances.
2) Metallurgy of alkali and alkaline earth metals.
3) Purification of metals.
4) Manufacture of pure gases.
5) Manufacture of compounds like sodium hydroxide, sodium carbonate, potassium chlorate etc.
6) Electroplating for corrosion resistance, ornaments etc.